This 75-minute webinar is centred around data from trials investigating the efficacy and safety of aducanumab, an investigational treatment for early Alzheimer’s disease. Featured in the webinar is ADI’s Chief Executive Paola Barbarino; Dr. Alireza Atri, Chair of ADI’s Medical and Scientific Advisory Panel and Biogen’s Head of Neurodegeneration, Dr. Samantha Budd Haeberlein.
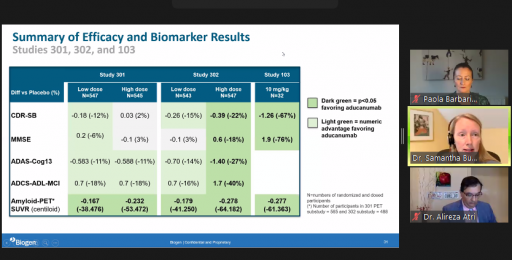 In the webinar, Dr. Haeberlein presents the data from Biogen’s Phase 3 clinical studies ENGAGE and EMERGE. Dr Haeberlein also goes into detail as to why Biogen’s Phase 3 clinical trials for the investigative Alzheimer’s drug was prematurely terminated in 2019 and how the full data set provides evidence of clinical effectiveness.
In the webinar, Dr. Haeberlein presents the data from Biogen’s Phase 3 clinical studies ENGAGE and EMERGE. Dr Haeberlein also goes into detail as to why Biogen’s Phase 3 clinical trials for the investigative Alzheimer’s drug was prematurely terminated in 2019 and how the full data set provides evidence of clinical effectiveness.
As of January 2021, aducanumab is currently under regulatory review in the United States, the European Union and Japan. If effective, the investigational drug would be the first disease-modifying treatment for Alzheimer’s disease.


